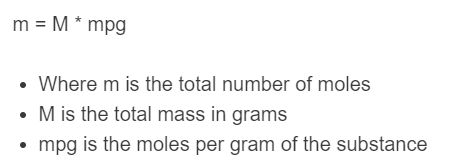Enter the total number of grams of a substance and the moles per gram of that substance to calculate the total number of moles
- Mole Fraction Calculator
- Molality Calculator
- Partial Pressure Calculator
- Moles to Molecules Calculator
Grams to moles formula
The following formula is used to convert the total number of grams of a substance to the total number of moles.
m = M * mpg
- Where m is the total number of moles
- M is the total mass in grams
- mpg is the moles per gram of the substance
For this formula, the mass and moles per unit pass could be switched to any unit you have. For example, if you have a mass of 2 lbs, you must calculate or determine the number of moles per pound, and then the total number of moles can be calculated.
Grams to Moles Definition
Converting grams to moles is as simple as multiplying the total mass by the moles per unit of mass.
How to convert grams to moles
The following is a step-by-step guide on how one might convert the total amount of grams of a substance to the total number of moles. For this example, we will assume we have a solution with two different substances, at 65% and 35% concentration by weight.
- The first step is to calculate the total number of grams for the substance we want to convert to moles. It’s assumed that the total weight is 100 grams and we are trying to find the weight of the 65% concentration by weight substance. Therefore, we must multiply 100*65% = grams.
- Next, the total number of moles per gram of the substance must be either calculated or measured. This is solely determined by the elements in the substance. The learn more about how this is calculated, visit this blog post. For this example, we are going to assume that we know this to be 1000 moles per gram. Note that this number is very very low, and only used to make this simpler.
- The final step is the plug the information we gathered above into the formula, or calculator if you’re feeling lazy. We find m = 65 * 1000 = 65,000 moles.
- Analyze the results to see if the answer makes logical sense. if this was a real problem, in this step you would find that the answer does not make logical sense because the total moles in a gram of substance should be orders of magnitude greater than what it was in this problem.
FAQ
What is the significance of converting grams to moles in chemistry?Converting grams to moles is crucial in chemistry because it allows scientists to quantify and compare amounts of different substances on a molecular level, facilitating stoichiometric calculations and chemical reactions analysis.
How do you find the moles per gram of a substance?The moles per gram of a substance can be found by taking the reciprocal of the molar mass of the substance. The molar mass, usually expressed in grams per mole, can be calculated by summing the atomic masses of all atoms in a molecule of the substance.
Can the grams to moles conversion be used for any substance?Yes, the grams to moles conversion can be applied to any substance, provided you know the substance’s molar mass or can calculate the moles per gram. This conversion is a fundamental principle in chemistry that is universally applicable.
Why is it important to analyze the results of a grams to moles conversion?Analyzing the results is important to ensure the conversion makes logical sense and the calculations are correct. This step helps identify potential errors in the calculation process and verifies the practicality of the results in real-world applications.