Frequently Asked Questions
The frequency factor (A) is a pre-exponential factor in the Arrhenius equation, representing the rate constant at which molecules collide with the correct orientation for a reaction to occur. It has the same units as the rate constant (sec^-1).
Activation energy (Ea) is the minimum amount of energy required for a reaction to proceed. A higher activation energy means that fewer molecules possess the necessary energy for the reaction, resulting in a slower reaction rate. A lower activation energy allows more molecules to react, increasing the reaction rate.
Temperature (T) is crucial in the Arrhenius equation because it affects the rate constant of a reaction. As temperature increases, the number of molecules with enough energy to overcome the activation energy barrier also increases, leading to a faster reaction rate. The temperature must be measured in Kelvin for the equation.
The gas constant (R) in the Arrhenius equation is a universal constant with a value of 8.314 * 10^-3 kj mol^-1 K^-1. It appears in many thermodynamic and kinetic equations and serves as a proportionality constant between energy, temperature, and the number of particles in a system.
Yes, the Arrhenius equation can be applied to non-gaseous reactions as well. Although the equation was initially developed for gas-phase reactions, it has been found to be applicable to reactions in various phases, such as liquid or solid, as long as the rate constant, activation energy, and temperature are appropriately defined.
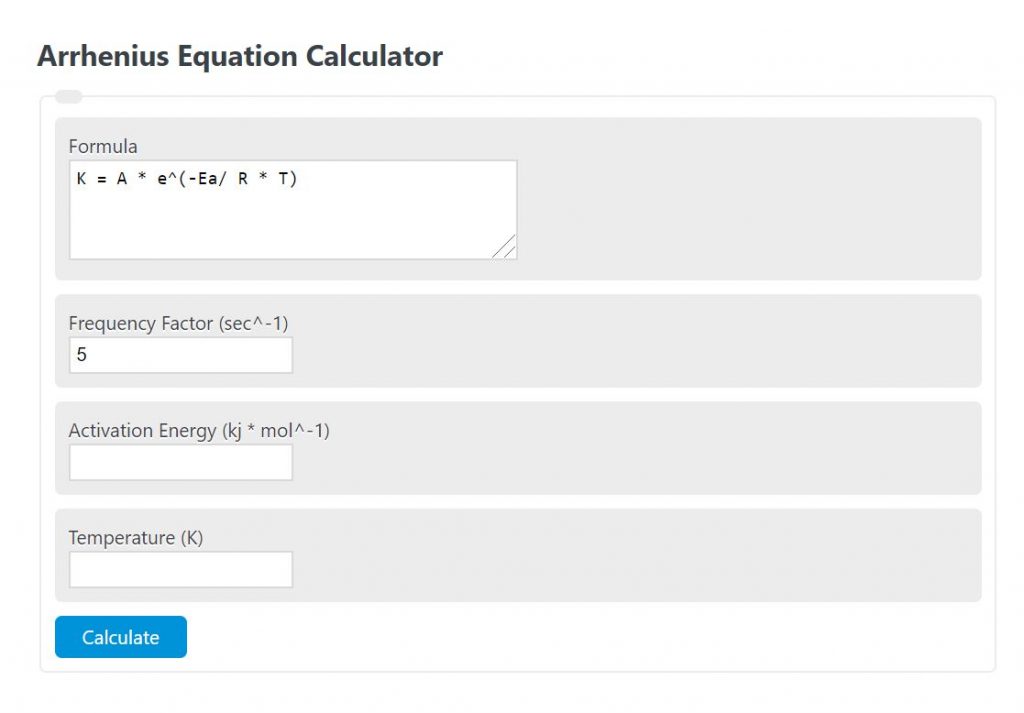
Enter the frequency factor, activation energy, and temperature into the calculator to determine the rate constant using the Arrhenius equation.
- Activation Energy Calculator
- Ideal Gas Law Calculator
- Charle's Law Calculator
- Equilibrium Constant Calculator
Arrhenius Equation
The following formula is used to calculate the rate constant of a reaction using the Arrhenius equation.
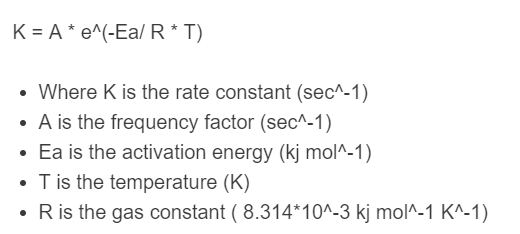
K = A * e^(-Ea/ R * T)
- Where K is the rate constant (sec^-1)
- A is the frequency factor (sec^-1)
- Ea is the activation energy (kj mol^-1)
- T is the temperature (K)
- R is the gas constant ( 8.314*10^-3 kj mol^-1 K^-1)
Arrhenius Equation Definition
The Arrhenius equation describes the relationship between the rate constant of a chemical reaction and the temperature, activation energy, and frequency factor.
Arrhenius Equation Example
How to calculate rate constant using Arrhenius equation
- First, determine the frequency factor.
Calculate or measure the frequency factor of the reaction.
- Next, determine the activation energy.
You can use the calculator linked above to determine the activation energy of the reaction.
- Next, measure the temperature.
Measure the temperature of the reaction in Kelvin.
- Finally, calculate the rate constant.
Calculate the rate constant using the formula above and values from steps 1-3.
The Arrhenius equation is a formula used to relate and calculate the rate constant of a chemical reaction to the temperature, activation energy, and frequency factor.
A rate constant is a constant that measures the rate at which a chemical reaction takes place.