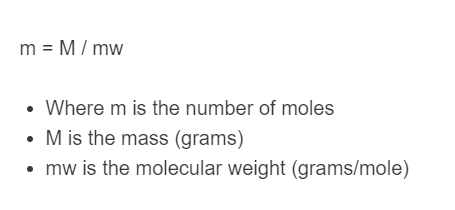Enter the total number of grams of the substance and the molecular weight into the calculator to determine the total number of moles.
- Moles to Atoms Calculator
- Grams to Moles Calculator
- mg to ml Calculator
- Moles to Molecules Calculator
- mEq Calculator
- Moles Per Gram Calculator
Mol Formula
The following formula is used to calculate the total number of moles of a substance.
m = M / mw
- Where m is the number of moles
- M is the mass (grams)
- MW is the molecular weight (grams/mole)
To calculate moles, divide the mass by the molecular weight.
Mol Definition
A mole is a unitless measure that describes the number of atoms in a particular amount of substance.
Mol Example
How to calculate the number of moles?
The following example problem explores the steps required to calculate the number of moles of a substance given the mass and the molecular weight of that substance.
The first step is to determine the total mass of the substance. For this example, the total mass is measured to be 500 grams.
Next, the molecular weight must be determined. For this example, the substance being analyzed is water. The molecular weight of water is known to be 18.01528 grams/mol.
Finally, calculate the number of moles of water using the formula above:
m = M / mw
m = 500 / 18.01528
m = 27.754 moles of water.
FAQ
A mole is a unitless measure that describes a certain number of atoms in a substance. To calculate the number of moles from atoms, navigate to the calculator linked above.
Molecular weight is a measurement that is similar to density. Instead of mass per unit volume, it’s a mass per mole.