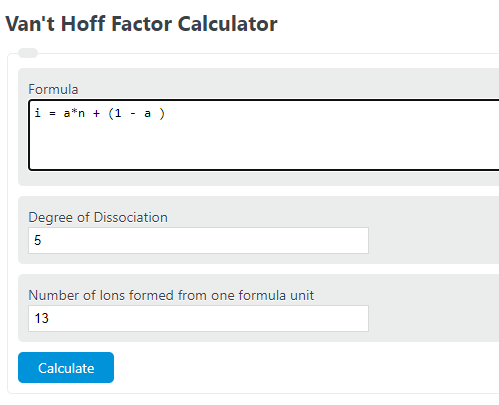
Enter the degree of dissociation and the number of ions formed from one formula unit of the substance to determine the Van’t Hoff Factor.
- Rate of Reaction Calculator
- Bond Order Calculator
- Mass Molarity Calculator
- Freezing Point Depression Calculator
- Ionization Energy Calculator
Van’t Hoff Factor Formula
The following formula can be used to calculate a Van’t Hoff Factor.
i = a*n + (1 - a )
- where i is the Van’t Hoff Factor
- a is the degree of dissociation
- n is the number of ions formed from one formula unit of the substance.
To calculate the Van’t Hoff Factor, multiply the degree of dissociation by the number of ions formed from one formula unit, then add a value of 1 minus the degrees of dissociation to the previous result.
Van’t Hoff Factor Definition
The Van’t Hoff Factor is the ratio of the actual concentration of particles produced when a substance is dissolved and the concentration of a substance as calculated from its mass.
Van’t hoff Factor Example
How to calculate Van’t Hoff Factor?
- First, determine the degree of dissociation.
Calculate or measure the total degree of dissociation.
- Next, determine the number of ions formed.
Calculate the number of ions formed per one unit of substance.
- Finally, calculate the Van’t Hoff Factor.
Calculate the factor using the equation above.
FAQ
A Van’t Hoff Factor is defined as the measure of the effect of a solute on the colligative properties such as osmotic pressure.