Enter the percentage abundance and mass of up to 5 different isotopes into the average atom mass calculator. The calculator will display the average atomic mass of the isotopes.
- All Mass Calculators
- Grams to Moles calculator
- Mole Fraction Calculator
- Gibbs Free Energy Calculator
- Bond Order Calculator
Average Atomic Mass Formula
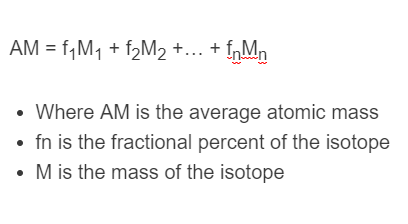
The following formula is used to calculate the average atomic mass of a substance.
AM = f_1M_1 + f_2M_2 +… + f_nM_n
- Where AM is the average atomic mass
- fn is the fractional percent of the isotope
- M is the mass of the isotope
To calculate the average atomic mass, multiply the fractional percent of each isotope by the mass of the isotope, then sum these values together.
Average Atomic Mass Definition
An average atomic mass is defined as the average mass of all isotopes within a given substance.
How to calculate average atomic mass?
How to calculate average atomic mass?
- First, determine the fractional percent of each isotope in the substance
For example, chlorine has two major isotopes. 1 with 75.77 percent of atoms and 1 with 24.23 percent of atoms. These two percentages would be the fractional percents of those isotopes.
- Next, determine the masses of each isotope
Using the same example above, this would be 35 and 37 amu respectively.
- Finally, calculate the average atomic mass
Calculate the average atomic mass using the information from steps 1 and 2 and the formula above.
FAQ
The average atomic mass is the average mass of all of the isotopes that make up a substance.
The fractional percent is the total percentage of a particular isotope in a substance.
“““““““““““““““““““““““““““““““““““““““““““““““`