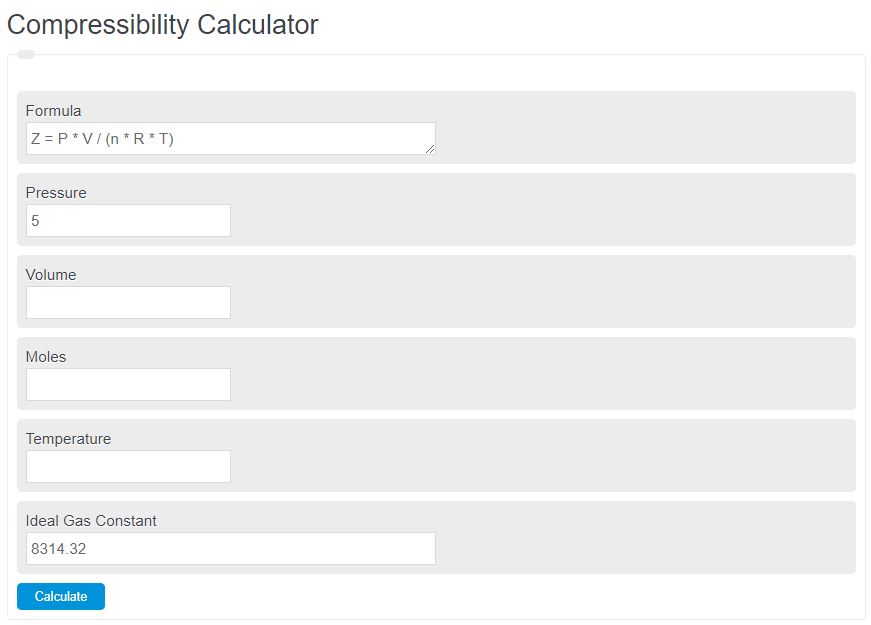
Enter the pressure, volume, number of moles, and temperature into the calculator to determine the compressibility factor of a non-ideal gas.
- Compression Ratio Calculator
- Ideal Gas Law Calculator
- Partial Pressure Calculator
- Pressure Altitude Calculator
Compressibility Formula
The following formula is used to calculate the compressibility factor of a gas.
Z = P * V / (n * R * T)
- Where Z is the compressibility factor
- P is the pressure (pascals)
- V is the volume (m^3)
- n is the number of moles
- R is the gas constant (8.31432*10^3)
- T is the temperature (K)
Pressure is the force applied per unit area, measured in pascals.
Volume is a measure of the amount of space occupied by a three-dimensional object.
Number of moles is a unit that represents the amount of a substance, equal to the Avogadro constant (6.022 x 10^23) times the mass of the substance in grams.
The gas constant is a mathematical constant used in the ideal gas law to relate the properties of gases, such as pressure, volume, and temperature.
Temperature (K) measures the average kinetic energy of the particles in a substance or system, expressed in Kelvin.
What is a Compressibility?
Compressibility is a property that pertains to the ability of a substance to be compressed or squeezed into a smaller volume when subjected to external forces. It is observed in various materials, including gases, liquids, and solids.
When discussing compressibility, we refer to how easily a substance can be pressed or compacted. Think of it as if you were trying to squeeze a sponge. If the sponge easily gets smaller when you apply pressure, then it is highly compressible. On the other hand, if the sponge remains firm and doesn’t change its volume much, it is less compressible.
In gases, compressibility is particularly evident. Gases consist of molecules that are constantly moving and colliding with each other. When pressure is applied to a gas, its molecules get closer together, reducing the space between them and causing the gas to occupy a smaller volume. This is why, for instance, we can store large amounts of compressed gas in small containers like aerosol cans.
Liquids also possess some degree of compressibility, although it is usually much lower than gases. When pressure is applied to a liquid, its molecules can come closer together to some extent, resulting in a slight reduction in volume. However, this change is usually very small and not easily noticeable in everyday situations.
Solids, like metals or rocks, are generally considered to be incompressible. When pressure is applied to a solid, its atoms or molecules are closely packed, leaving little room for further compression. This is why solid objects, such as a brick or a table, do not change their volume significantly when we push or apply force to them.
Understanding the concept of compressibility helps us design and engineer various systems and devices. For example, it plays a crucial role in designing engines or hydraulic systems, where the behavior of compressible fluids, such as air or oil, needs to be precisely controlled.
Compressibility Example
How to calculate compressibility?
- First, determine the pressure.
Measure the pressure acting on the material.
- Next, determine the volume of the material.
Calculate the total volume of the gas under that pressure.
- Next, determine the number of moles.
Calculate the total number of moles of the gas.
- Next, determine the temperature.
Calculate or measure the temperature of the gas.
- Finally, calculate the compressibility factor.
Calculate the compressibility factor using the equation above.
FAQ
Compressibility is a measure of a material or gasses ability to compress under a certain force or pressure.
This factor is a ratio of the pressure and volume to the moles and temperature of a gas. It represents the compressibility of the gas.
Compressibility is calculated using the ideal gas formula and re-arranging the values to create a ratio.