Calculate the temperature of a substance given the pressure, volume, and amount of substance through the ideal gas law.
- Pressure Calculator
- Partial Pressure Calculator
- Boyle’s Law Calculator
- Atmospheric Pressure Calculator
- Volume at Standard Temperature and Pressure Calculator
- Tire Pressure Temperature Calculator
- Chimney Draft Calculator
Ideal Gas Law Formula
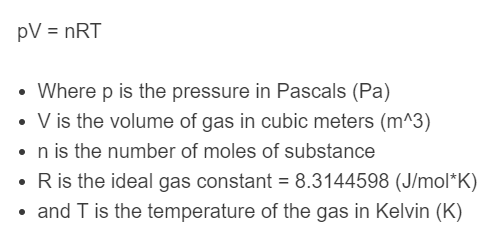
The following equation is used to define ideal gasses:
pV = nRT
- Where p is the pressure in Pascals (Pa)
- V is the volume of gas in cubic meters (m^3)
- n is the number of moles of substance
- R is the ideal gas constant = 8.3144598 (J/mol*K)
- and T is the temperature of the gas in Kelvin (K)
Ideal Gas Definition
An ideal gas is a form of gas that follows a set of conditions. These conditions mean that the ideal gas can be evaluated in simplified conditions that help with modeling and solving equations. The following is the set of parameters that define an ideal gas
- They obey Newton’s laws of motion
- They don’t interact except with collisions. I.E. no chemical reactions
- Collisions are elastic
- They are modeled as point particles (take up no space)
- It’s a large amount of substance.
If all of these criteria are met, then this gas can be modeled by the ideal gas law and corresponding equation.
How to calculate ideal gas properties
The properties of a so-called ideal gas all follow the equation outlined above. Since the gas constant is already known, only 3 out of 4 properties must be known in order to calculate the missing property. For instance, if you know the pressure, volume, and amount of substance, you can calculate the temperature. If you know the pressure, temperature, and volume, you can calculate the amount of substance. Simply re-arrange the equation, and you are off and running.
The following example will go over the steps to calculating the properties of an ideal gas. In this case, specifically, we will solve for pressure.
- First, we must rearrange the ideal gas equation to solve for pressure. We can simply do this by dividing both sides by volume. The result is the following: p = nrT/V.
- Now that we have pressure solved for in the equation, we need to find or measure the 3 other variables. This would be the number of moles, temperature, and volume. For this example, we will assume 1 mole, 1 degree C temperature, and 1 cubic meter of volume.
- Finally, enter all of that information into the formula, and you can find your pressure in pascals.
FAQ
What is the ideal gas constant, and why is it important?
The ideal gas constant, denoted as R, is a physical constant that appears in the equation of state of ideal gases. Its value is approximately 8.3144598 J/(mol·K). It is important because it relates the energy scale to the temperature scale in the context of an ideal gas, allowing the ideal gas law to be applied in practical situations, such as calculating the pressure, volume, temperature, or amount of an ideal gas.
How do real gases differ from ideal gases?
Real gases differ from ideal gases in several ways. Ideal gases are theoretical constructs that follow the ideal gas law under all conditions. In contrast, real gases deviate from the ideal gas behavior at high pressures and low temperatures. This deviation occurs because real gases have intermolecular forces and their molecules occupy space, whereas ideal gases are assumed to have no intermolecular forces and their molecules do not occupy space. These differences become significant under conditions where gases are compressed to high densities or cooled to low temperatures.
Can the ideal gas law be applied to mixtures of gases?
Yes, the ideal gas law can be applied to mixtures of gases. This is done through Dalton’s Law of Partial Pressures, which states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of each individual gas in the mixture. Each gas in the mixture is considered to behave as an ideal gas and contributes to the total pressure in proportion to its fraction of the total number of moles of gas present. This allows for the calculation of properties of gas mixtures using the ideal gas law, provided the conditions are such that the gases behave ideally.