Enter the charge of object 1, the charge of object 2, and the distance between them to calculate the electric force between them. Calculate the attractive or repulsive electric force between two objects.
- Electric Field Calculator
- Lorentz Force Calculator
- Acceleration in an electric field calculator
- Magnetic Flux Calculator
- Electromotive Force Calculator
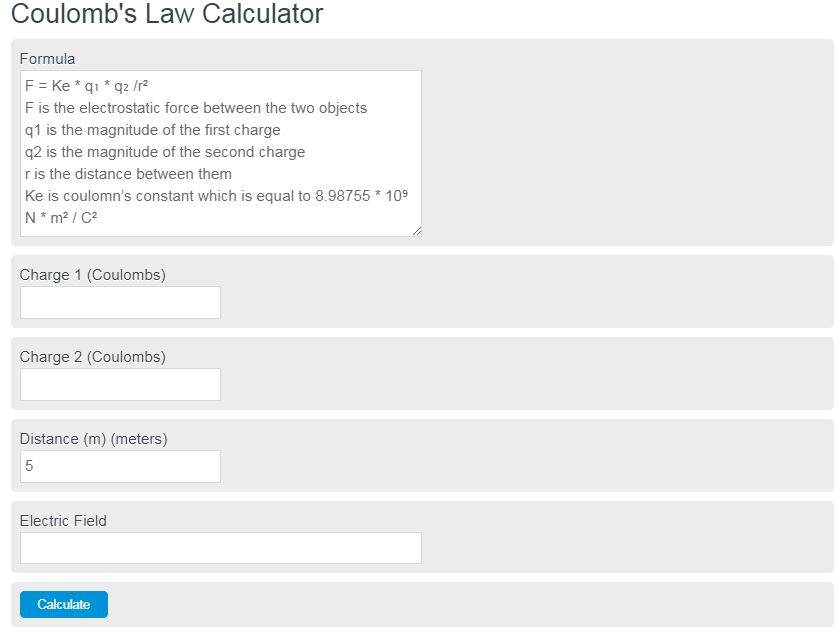
Coulomb’s Law Formula
The formula for the electric force between two charged particles is as follows:
F = Ke * q₁ * q₂ /r²
- F is the electrostatic force between the two objects
- q1 is the magnitude of the first charge
- q2 is the magnitude of the second charge
- r is the distance between them
- Ke is Coulomb’s constant which is equal to 8.98755 * 10⁹ N * m² / C²
Coulomb’s Law Definition
Coulomb’s law is also known as the inverse-square law. It’s a law of physics that describes the force between two stationary particles. These particles, of course, need to be charged, or there would be no force between them. This force is known as the electrostatic effect. This law was fundamental to the development of the theory of electromagnetism.
How to calculate Coulomb’s law?
How to calculate an electric force
- First, determine the charges of both points.
The charge of each object must first be measured. For this example, we will say both points hold a charge of 10 coulombs.
- Next, find the distance between the object
The distance between the objects should be the shortest point between them. For this example, we will assume 10 meters
- Finally, enter the information into the formula
Using the formula above we find the electric force is 8987550000 N.
FAQ
What determines if the electric force between two objects is attractive or repulsive?The nature of the electric force between two objects depends on the charges of the objects involved. If the charges are of opposite signs (one positive and one negative), the force is attractive. If the charges are of the same sign (both positive or both negative), the force is repulsive.
How does distance affect the magnitude of the electric force between two charged objects?According to Coulomb’s Law, the electric force between two charged objects is inversely proportional to the square of the distance between them. This means that as the distance between the two objects increases, the force decreases rapidly, and vice versa.
Can Coulomb’s Law be applied to charged objects in motion?Coulomb’s Law primarily describes the electrostatic force between two stationary charged particles. When dealing with moving charges, other factors such as magnetic forces and relativistic effects may need to be considered, which are beyond the scope of Coulomb’s Law.