Enter the elementary charge and the extra number of electrons into the calculator to determine the net charge. This calculator can also determine the number of electrons gained or lost when given the other variables.
- All Charge Calculators
- Effective Nuclear Charge Calculator
- Bond Length Calculator
- Formal Charge Calculator
- Drift Velocity Calculator
- Refrigerant Charge Calculator
- Charge Force Calculator
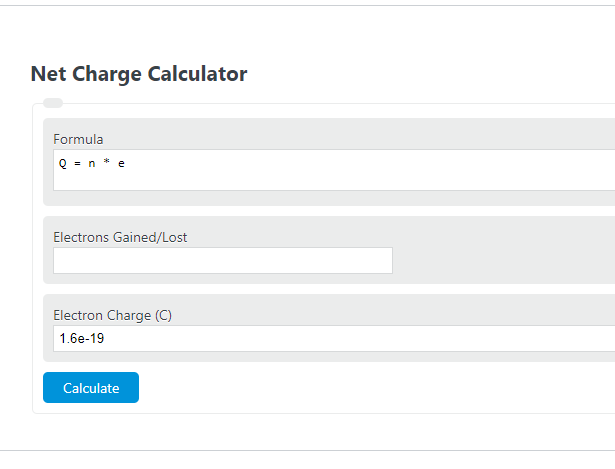
Net Charge Formula
The following formula is used to calculate a net charge.
Q = n * e
- Where Q is the net charge (C)
- n is the number of extra electrons gained or lost
- e is the charge of one electron (1.6*10^-19 C)
To calculate a net charge, multiply the number of electrons gained or lost by the charge of an electron.
Net Charge Definition
What is a net charge? A net charge is the total charge of an atom that has gained or lost electrons causing it to have either a positive or negative charge.
Example Problem
How to calculate net charge?
- First, determine the charge of one electron.
An electron charge is known to be approximately 1.6*10^-19 (C) where C is the units of Coulombs.
- Next, determine the number of electrons gained or lost.
For this problem, the atom has gained 2 electrons.
- Finally, calculate the net charge.
Using the formula above, the net charge is found to be 1.6*10^-19 * 2 = 3.2*10^-19.