Enter the covalent radii of two atoms and the electronegativity of each atom into the calculator to determine the bond length.
- Bond Order Calculator
- Effective Nuclear Charge Calculator
- Binding Energy Calculator
- Nuclear Q Value Calculator
Bond Length Formula
The following formula is used to calculate a bond length.
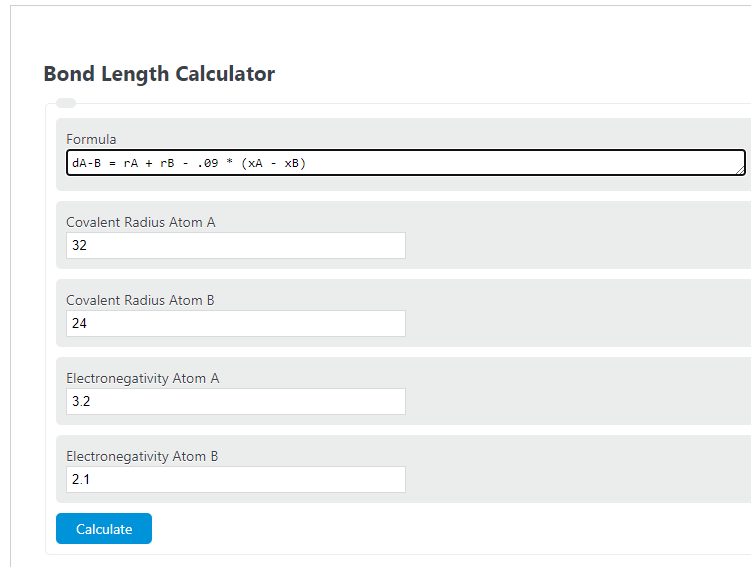
dA-B = rA + rB - .09 * (xA - xB)
- Where dA-B is the distance between the nuclei of atoms A and B
- rA is the covalent radius of atom A
- rA is the covalent radius of atom B
- xA is the electronegativity of atom A
- xB is the electronegativity of atom B
Bond Length Definition
What is a bond length? A bond length is a measure of the distance between the centers of two bonded nuclei. The distance is typically measured in a picometer.
Example
How to calculate bond length?
- First, determine the lengths of the covalent radii.
For this example, the radius of atom A is 24 picometers and the radius of atom B is 32 picometers.
- Next, determine the electronegativity of atom A.
For this problem, atom A has an electronegativity of 3.2.
- Next, determine the electronegativity of atom B.
The electronegativity of atom B is found to be 2.1.
- Finally, calculate the bond length.
Using the formula above, the bong length is found to be dA-B= 24+32-.09*(3.2-2.1) = 55.901.
About Bond Length
Is bond length an average? In general, when calculating a bond length, the resulting answer is an average length between nuclei.
How does bond length change with bond order? As bond order increases, the bond length will decrease. For example, a third bond order will have a smaller bond length than a second bond order.