Enter the number of ions produced from dissociation, osmotic coefficient, concentration, and temperature into the calculator to determine the osmotic pressure.
- Atmospheric Pressure Calculator
- Gauge Pressure Calculator
- Absolute Pressure Calculator
- Cell Density Calculator
Osmotic Pressure Formula
The following equation is used to calculate osmotic pressure.
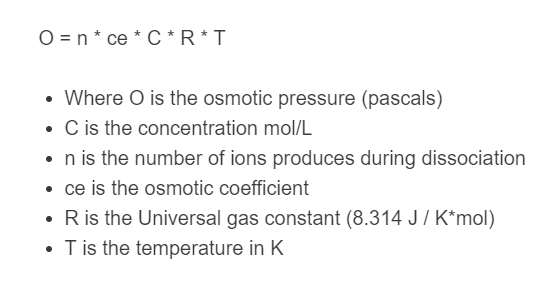
O = n * ce * C * R * T
- Where O is the osmotic pressure (pascals)
- C is the concentration mol/L
- n is the number of ions produced during dissociation
- ce is the osmotic coefficient
- R is the Universal gas constant (8.314 J / K*mol)
- T is the temperature in K
Osmotic Pressure Definition
Osmotic pressure is a concept in chemistry that describes the force exerted by a solvent when it flows through a semipermeable membrane to equalize the solute concentration on both sides.
It arises due to the random movement of solvent molecules and their interactions with solute particles.
Osmotic pressure is the pressure required to prevent the flow of solvent molecules into a solution through a membrane. When a solution is separated from its pure solvent by a semipermeable membrane, the solvent molecules move across the membrane to dilute the higher solute concentration.
Osmotic Pressure Example Problem
The following is an example problem of how to calculate an osmotic pressure.
First, we must determine the concentration of the solution. Through analysis, we determine that this value is 10 mol/L of NaCl.
Next, we need to determine the number of ions produced by dissociation. Using a lookup table we see that NaCl has a value of 52.
Next, we determine the osmotic coefficient through experimental data and find it to be .93.
Next, determine the temperature during the osmotic process. It’s determined that this value is 100K.
Finally, using the information we gathered and the equation presented above, we determine the osmotic pressure to be 402,065 Pa.
FAQ
Osmotic pressure is the pressure required to prevent the flow of a solution through a membrane. It’s often described as the “minimum” pressure to stop the process of osmosis from occurring.
The osmotic coefficient is a value derived from the type of solution that’s attempting to go through osmosis. For NaCl it is equal to .93 and for KCl it is equal to .92.