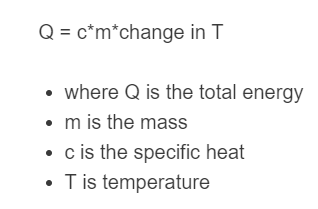Enter the total amount of energy applied to the object, the change in temperature of that object, and the mass of the object to calculate the specific heat.
- Enthalpy Calculator
- Efficiency Calculator
- Ideal Gas Law Calculator
- Science & Engineering Calculators
Specific Heat Formula
Specific heat is governed by the following thermodynamic equation for heat capacity:
Q = c*m*change in T
c = Q/m*T
- where Q is the total energy
- m is the mass
- c is the specific heat
- T is temperature
To calculate the specific heat, divide the total energy by the mass, then multiply by the change in temperature.
Specific Heat Definition
Specific heat is a fundamental property of a substance that measures its ability to absorb or release heat energy when the temperature changes. It quantifies the amount of heat energy required to raise the temperature of a given amount of a substance by a certain degree. In other words, specific heat represents the amount of energy needed to heat a material.
Specific heat is crucial because it helps us understand how different substances respond to changes in temperature. By knowing the specific heat of a substance, we can predict how it will behave when exposed to heat or cold.
For example, substances with low specific heat require less energy to heat up, making them heat up quickly and cool down rapidly. On the other hand, substances with high specific heat need more energy to heat up, so they heat up slowly and retain heat for longer periods.
This property has various practical applications. For instance, it helps in designing efficient heating and cooling systems for buildings. By understanding the specific heat of different materials used in construction, engineers can determine the amount of energy required to heat or cool a room, ensuring optimal temperature control while minimizing energy waste.
Additionally, specific heat is crucial in determining proper insulation materials that can reduce heat transfer and minimize energy consumption.
How to calculate specific heat?
You could just simply use the calculator above, but it’s almost always better to understand a calculation so you can tweak the result.
First, you need to determine the total amount of energy being put into the system. Sometimes this can be measure directly, other times it needs to be calculated. For this example, the total energy is found to be 100 Joules.
Second, you need to measure the mass of the object you are testing/calculating. For this example, the object is a ball of unknown material that weighs 20 kg.
Third, you need to measure the change in temperature of the object. The change in temperature is found to be 40C when 100 Joules of energy is added to the ball.
Finally, the specific heat can be calculated using the formula above. The final value is found to be 100*20*40 =.125 J/kg *C.
Specific Heat Units
The standard international units for specific heat capacity are joules per kelvin per kilogram. This is denoted J/K/kg or J/(K*kg).
The metric units used for specific heat are calories per gram per degrees Celsius. This is denoted cal/g/C or cal/(g*C).
The English system of units for specific heat are Btu/lb*f, or British Thermal Unit per pound per degrees Fahrenheit.
High Specific Heat Materials
The following list outlines examples of 10 different materials with some of the highest heat capacities known to humans.
- Hydrogen – 14.30 J/(g*K)
- Helium – 5.1932 J/(g*K)
- Ammonia – 4.700 J/(g*K)
- Lithium – 4.379 J/(g*K)
- Water – 4.1813 J/(g*K)
- Ethanol – 2.44 J/(g*K)
- Polyethylene – 2.3027 J/(g*K)
- Gasoline – 2.22 J/(g*K)
- Methane – 2.191 J/(g*K)
- Methanol – 2.14 J/(g*K)
Low Specific Heat Materials
This list provides examples of known materials with some of the lowest specific heats on Earth.
- Uranium – .116 J/(g*K)
- Bismuth – .123 J/(g*K)
- Gold – .129 J/(g*K)
- Lead – .129 J/(g*K)
- Tungsten – .134 J/(g*K)
- Mercury – .1395 J/(g*K)
- Antimony – . 207 J/(g*K)
- Tin – .227 J/(g*K)
- Cadmium – .231 J/(g*K)
- Silver – .233 J/(g*K)
Specific Heat Properties
Specific heat is a fundamental property of all matter that describes how much energy it takes to increase the temperature of a material.
High specific heat would mean that it takes a lot of energy to increase the temperature of the material. For example, water has a very high specific heat.
A low specific heat would mean it takes very little energy to increase the temperature of the material. For example, uranium has a very low specific heat at about .116 J/(g*K).
Molar Heat Capacity
Molar heat capacity, or sometimes referred to as molar specific heat, is a measure of the specific heat per unit of 1 mole of material.
Molar heat capacity is used to understand the specific heat of a material with respect to a number of atoms. This is useful when looking at different materials since they vary in density.
FAQ
Specific heat is a measure of the amount of heat or energy needed to raise the temperature of a material or object by 1 degree Celsius.