Enter the change in internal energy, the change in volume, and the change in pressure of a reaction to calculate the total change in Enthalpy. This calculator can also evaluate the change in energy or change in volume given the other known values.
- All Enthalpy Calculators
- Change in Enthalpy Calculator
- Specific Heat Calculator
- Efficiency Calculator
- Entropy Calculator
- Heat of Fusion Calculator
- Specific Enthalpy Calculator
Enthalpy Formula
Enthalpy is defined as the heat absorbed by a system and the total work done while expanding. The following formula can be used.
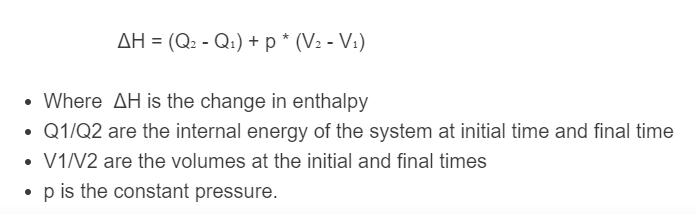
ΔH = (Q₂ - Q₁) + p * (V₂ - V₁)
- Where ΔH is the change in enthalpy
- Q1/Q2 is the internal energy of the system at an initial time and final time
- V1/V2 are the volumes at the initial and final times
- p is the constant pressure.
To calculate enthalpy, also known as a change in enthalpy or delta h, take the difference in internal energy, then add the pressure multiplied by the change in volume to the result.
Enthalpy Definition
Enthalpy is a measure of total energy in a system. This is typically in the form of heat, but also a form of volume and pressure. Since enthalpy is is a measure of the state of a system, it does not change at equilibrium.
That is why we look at the change in enthalpy of a system from one state to another. The state of the system has to change in order for the enthalpy to change. This normally happens when work or energy is transferred to a system, typically through heat.
Enthalpy changes in both endothermic or exothermic reactions. An endothermic reaction is an act of absorbing energy to change states, and an exothermic reaction is an act of releasing energy or heat. The change in enthalpy will be positive for endothermic and negative for exothermic.
How to calculate Enthalpy?
How to calculate Enthalpy
- First, determine the initial energy of the reaction
With this step, we must calculate or measure the initial energy of the system, Q1. For this example, we will assume a value of 20 joules.
- Next, determine the initial volume of the substance
Measure the initial volume empirically, or through calculations. We will assume 1 cubic meter.
- Next, measure the constant pressure of the system/substance
The pressure of this reaction must be constant for the formula to work. Therefore, the pressure measurement will be the same at the start and end of the reaction. We will assume 1 pascal.
- Now, let the reaction occur.
Once the reaction has occurred, measure the final volume and energy of the substance. For this example let’s say this is 10 joules and .5 cubic meters respectively.
- Finally, calculate the results
Enter the known information into the formula or calculator above to determine the change in enthalpy. Through the formula, we find that the change in enthalpy is 10.5 joules.
- Analyze the results
Analyze the result for accuracy and learn from this process.
FAQ
Enthalpy is a measure of total energy in a system. This is typically in the form of heat, but also a form of volume and pressure.