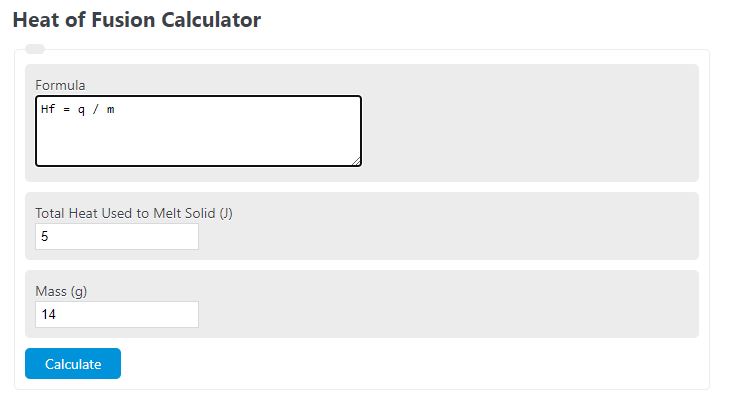
Enter the mass of a substance that was changed to a liquid state and the total heat required to determine the heat of fusion.
- Latent Heat Calculator
- Heat Load Calculator
- Heat of Combustion Calculator
- Heat of Vaporization Calculator
- Ice Melting Time Calculator
- Water Freeze Time Calculator
- Phase Change Energy Calculator
- Heat Capacity Ratio Calculator
Heat of Fusion Formula
The following formula is used to calculate a heat of fusion.
Hf = q / m
- Where Hf is the heat of fusion (J/g)
- q is the total heat required to melt the solid (J)
- m is the total mass of the material that was melted (g)
To calculate heat of fusion, divide the total heat required to melt the solid by the mass of the melted material.
Heat of Fusion Definition
The heat of fusion is defined as the total energy per unit mass required in order to turn a solid into a liquid of a given material.
Heat of Fusion Example
How to calculate heat of fusion?
- First, determine the total mass of solid that was melted.
For this example, the mass of the solid was determined to be 50g.
- Next, determine the total heat required to melt the solid.
After an experiment to melt the solid, it’s determined that the total heat added to the solid was 4000J.
- Finally, calculate the heat of fusion.
Using the formula we find the heat of fusion to be 4000J/50g = 80J/g.
FAQ
Heat of fusion is a measure of the amount of energy per unit mass that is required to melt a solid and turn it into a liquid.