Enter the mass of an object and the specific latent heat of that object to calculate the latent heat in Joules.
- Specific Heat Calculator
- Enthalpy Calculator
- Heat Load Calculator
- Heat Loss Calculator
- Heat Dissipation Calculator
- Steam Condensation Calculator
- Phase Change Energy Calculator
- Heat Exchanger Duty Calculator
Latent Heat Formula
The following equation can be used to calculate the latent heat of any object or material.
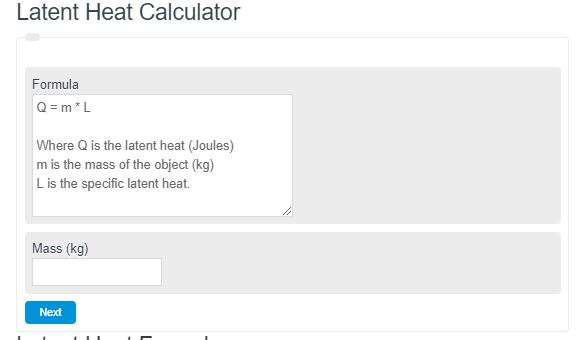
Q = m * L
- Where Q is the latent heat (Joules)
- m is the mass of the object (kg)
- L is the specific latent heat.
To calculate latent heat, multiply the mass of the object by the specific latent heat.
Just as with specific heat, latent heat can be calculated through the mass and a constant that measures the object’s ability to absorb heat. In the cases of latent heat, this is only the object’s ability to absorb heat during phase transitions.
Latent Heat Definition
In other words, when i substance such as water is boiled, the temperature of the water does not change when it’s undergoing the phase change to a gas. Only when the water is in a gaseous state does the temperature start to increase. The energy that is dumped into the water during this time is considered to be the latent heat.
How to calculate latent heat?
How to calculate latent heat?
- First, determine the mass of the substance.
Measure or calculate the total mass of the substance or object.
- Next, determine the specific latent heat.
Measure or look up the specific latent heat of the material.
- Finally, calculate the latent heat.
Using the formula above calculate the latent heat.
FAQ
Latent heat is the total energy required to change the phase of a substance. For example, changing water from a liquid to gas via boiling.