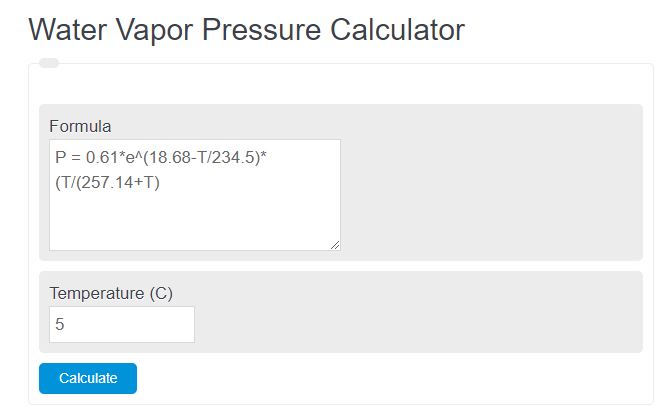
Enter the temperature in Celsius into the calculator below to determine the water vapor pressure.
- Vapor Pressure Calculator (Solvent/Solution)
- Water Pressure Calculator
- Pressure Altitude Calculator
- VPD (Vapor Pressure Deficit) Calculator
- Water Vapor Transmission Rate Calculator
Water Vapor Formula
The following formula is used to calculate the saturated water vapor pressure.
P = 0.61*e(18.68-T/234.5)*(T/(257.14+T)
- Where P is the saturated vapor pressure (kPA)
- T is the temperature (C)
Water Vapor Pressure Definition
Water vapor pressure is the force exerted by water vapor molecules in the air.
It represents the partial pressure of water vapor in a mixture of gases. When water evaporates, its molecules escape into the air, creating water vapor. As these molecules move around, they collide with each other and with other molecules in the air, exerting a certain amount of pressure.
Water Vapor Pressure Example
How to calculate water vapor pressure?
- First, measure the temperature of the air.
If necessary, convert the temperature into units of Celsius.
- Next, enter the temperature into the formula.
Calculate the water vapor pressure using the temperature from step 1 and the formula above.
- Finally, analyze the results.
Do the results make logical sense? If so, you have your answer, if not, check the calculation.
FAQ
How does temperature affect water vapor pressure?
Temperature has a direct impact on water vapor pressure. As the temperature increases, the kinetic energy of water molecules increases, allowing more water molecules to escape into the air and thus increasing the water vapor pressure. Conversely, as the temperature decreases, the water vapor pressure decreases.
Why is understanding water vapor pressure important?
Understanding water vapor pressure is crucial for various scientific and practical applications, including weather forecasting, the study of climate change, designing HVAC systems, and in industries such as agriculture and food packaging where humidity control is vital.
Can water vapor pressure exceed atmospheric pressure?
Water vapor pressure is a component of atmospheric pressure and represents the partial pressure exerted by water vapor alone. It cannot exceed the total atmospheric pressure under natural conditions. However, in a closed system, water vapor pressure can reach or exceed the atmospheric pressure, leading to phenomena like boiling.