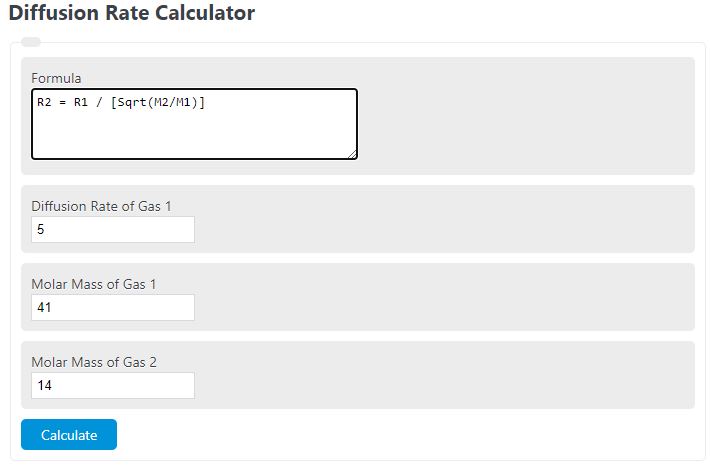
Enter the diffusion rate of a gas, its molar mass, and the molar mass of another gas to determine the diffusion rate of the second gas.
- Effusion Calculator
- Particles Velocity Calculator (Gas)
- Ideal Gas Law Calculator
- Diffusion Coefficient Calculator
Diffusion Rate Formula
The following formula is used to calculate the diffusion rate of a gas.
R2 = R1 / [Sqrt(M2/M1)]
- Where R2 is the diffusion rate of gas 2
- R1 is the diffusion rate of gas 1
- M2 is the molar mass of gas 2
- M1 is the molar mass of gas 1
To calculate a diffusion rate of a particular gas, divide the diffusion rate of the initial gas by the square root of the molar mass of gas 2 divide the molar mass of gas 1.
Diffusion Rate Definition
A diffusion rate is defined as the rate of movement of particles from a region of higher concentrations to a lower concentration.
Diffusion Rate Example
How to calculate diffusion rate?
- First, determine the diffusion rate of gas 1.
Measure the rate of diffusion of gas 1.
- Next, determine the molar masses.
Calculate the molar mass of both gas 1 and gas 2.
- Finally, calculate the diffusion rate.
Plug the values into the formula above to calculate the diffusion rate of the second gas.
FAQ
Diffusion is the movement of particles, often times a gas, from a region of a higher concentration to a lower concentration region.
Diffusion is used in fields such as physics, chemistry, biology, and more.