Enter the reaction rate, molar concentrations of A and B, and the partial orders of reaction of A and B to calculate the rate constant.
- Equilibrium Constant Calculator
- Rate of Reaction Calculator
- Decay Constant Calculator
- Reaction Velocity Calculator
- Standard Free Reaction Energy Calculator
Rate Constant Formula
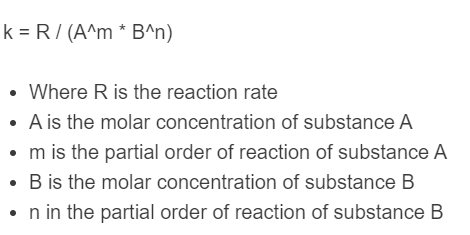
The following formula is used to calculate a rate constant.
k = R / (A^m * B^n)
- Where R is the reaction rate
- A is the molar concentration of substance A
- m is the partial order of reaction of substance A
- B is the molar concentration of substance B
- n in the partial order of reaction of substance B
To calculate a rate constant, divide the reaction rate by the product of the molar concentration of A raised to the power of the partial order reaction of A, times the molar concentration of B raised to the power of the partial order reaction of B.
Rate Constant Definition
A rate constant is a term used in chemistry to define the ratio or proportionality of the rate of reaction to the concentrations of the reactants.
Rate Constant Example
How to calculate a rate constant?
- First, determine the concentrations of A and B.
Calculate the molar concentration per unit volume of both reactants.
- Next, determine the partial orders of reaction.
Calculate the partial orders of reaction with respect to reactants A and B.
- Finally, calculate the rate constant.
Calculate the rate constant using the formula above.
FAQ
A reaction rate is the change in concentration of a reactant over time.