Enter the rate constant, the concentration of other species, the order of reaction with respect to A, and the order of reaction with respect to B into the calculator to determine the rate of reaction.
- Equilibrium Constant Calculator
- Cell Potential Calculator
- Extinction Coefficient Calculator
- Reaction Velocity Calculator
Rate of Reaction Formula
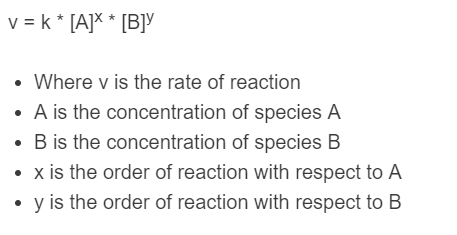
The following formula is used to calculate the rate of a reaction.
v = k * [A]^x * [B]^y
- Where v is the rate of reaction
- A is the concentration of species A
- B is the concentration of species B
- x is the order of reaction with respect to A
- y is the order of reaction with respect to B
- k is the rate constant
To calculate the rate of reaction, raise the concentration of species A to the order of reaction concerning A, then multiply the result by the concentration of species B to the order of reaction concerning B. Finally, multiply the result by the rate constant.
Rate of Reaction Definition
A rate of reaction is defined as the change in concentration over time of two or more reactants. It can also be considered the change in concentration of the chemical product of the reaction over time.
Rate of Reaction Example
How to calculate a rate of reaction?
- First, determine the concentration of species A and B.
Calculate the initial concentrations of both species.
- Next, determine the order of reactions.
Calculate the order of reactions with respect to A and the order with respect to B.
- Next, determine the rate constant.
Calculate or determine the rate constant.
- Finally, calculate the rate of reaction.
Calculate the rate of reaction using the equation above.
FAQ
A rate of reaction is a measure of the change in concentration over time of a given chemical interaction between two or more species.