Enter the mass and temperature of any gas into the calculator to determine the average velocity of the particles contained in that gas.
- Velocity Calculator
- Latent Heat Calculator
- Carnot Efficiency Calculator
- Kinetic Energy Calculator
- Thermal Energy Calculator
- Molecular Speed Calculator
- Superficial Gas Velocity Calculator
- Gas Velocity Calculator
- Thermal Velocity Calculator
Particles Velocity Formula
The following equation is used to calculate the average particle velocity of a gas at a certain temperature.
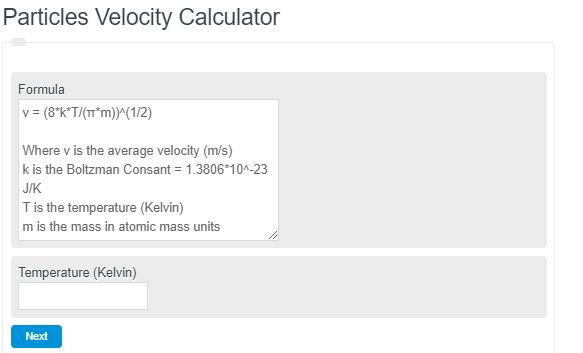
v = (8*k*T/(π*m))^{1/2}- Where v is the average velocity (m/s)
- k is the Boltzman Consant = 1.3806*10^-23 J/K
- T is the temperature (Kelvin)
- m is the mass in atomic mass units
To calculate the particle velocity, divide the product of 8 times the temperature times the Boltzmann constant by pi times the mass, then raise the result to the one-half power.
Particle Velocity Definition
This is also known as the Maxwell-Boltzmann Equation or distribution. This represents the average velocity of gas as a whole. Since temperature if the average kinetic energy of a gas, then calculating the velocity of any single particle using temperature would be impossible. Instead, we can calculate the average velocity since the temperature is also an average.
The larger the mass, the less the velocity, and vice versa with regard to temperature. Which makes logical sense. As temperature increases, kinetic energy increases.
How to calculate particle velocity?
How to calculate a particle velocity?
- First, determine the temperature of the gas.
Measure the temperature in Kelvin of the gas being analyzed.
- Next, determine the mass of the gas.
Measure the total mass of the gas in AMUs.
- Finally, calculate the particle velocity.
Using the formula above, along with the Boltzman constant, calculate the particle velocity.
FAQ
How does the mass of a gas affect its particle velocity?
The mass of a gas inversely affects its particle velocity. According to the Maxwell-Boltzmann distribution, a larger mass results in a lower average velocity of the gas particles. This is because the kinetic energy imparted to each particle is inversely proportional to its mass.
Why is the Kelvin scale used for temperature in the particle velocity formula?
The Kelvin scale is used because it is an absolute temperature scale where 0 Kelvin represents absolute zero, the theoretical point where particles have minimal kinetic energy. This scale directly relates to the kinetic energy of particles, making it essential for calculations involving particle velocity and energy.
Can the particle velocity formula be used for any gas?
Yes, the particle velocity formula can be applied to any ideal gas. Ideal gases are theoretical gases composed of point particles that interact according to the laws of physics but do not experience intermolecular forces. This formula may not accurately predict the behavior of real gases under high pressure or low temperature, where intermolecular forces become significant.